
Dosimeter badge services for medical, dental, and veterinary businesses
Learn how Radiation Detection Company’s easy-to-use dosimetry solutions can boost the efficiency of your practice.
Written by
Chris Passmore, CHP
President, Radiation Detection Company
Last Updated: October 30, 2025
The closer a dosimeter’s response stays to tissue equivalence, the more accurate, consistent, and trustworthy the dose measurement – and that makes all the difference in radiation safety.
Your body sees radiation one way. Your dosimeter might see it very differently.
When we measure radiation exposure, we’re not just counting particles – we’re trying to understand how that energy interacts with living tissue. That’s the foundation of dose measurement. In an ideal world, our detectors would respond exactly as human tissue does. In dosimetry, we call this tissue equivalence – a detector response ratio of 1.0.
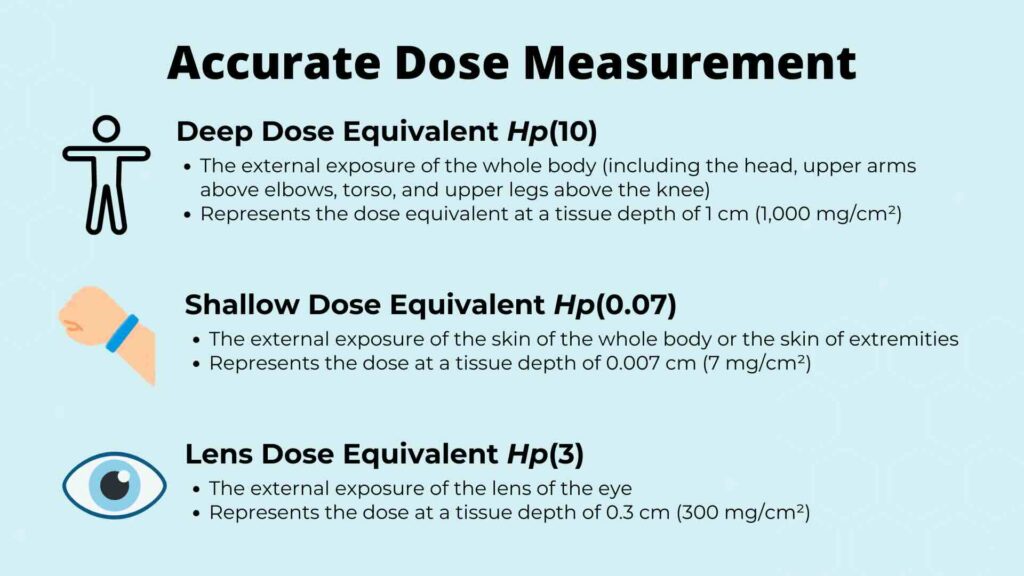
In reality, different detector materials “see” radiation differently depending on the type of energy measured, distance, and dosimeter material. That variation affects how accurately we can reconstruct the dose a person actually receives.
The further a dosimeter material strays from tissue equivalence, the more complicated the dosimeter becomes.
Radiation dose measurement is fundamentally about quantifying energy deposition in tissue. The absorbed dose represents energy per unit mass, but personnel dosimetry focuses on dose equivalent, accounting for radiation type and biological effectiveness.
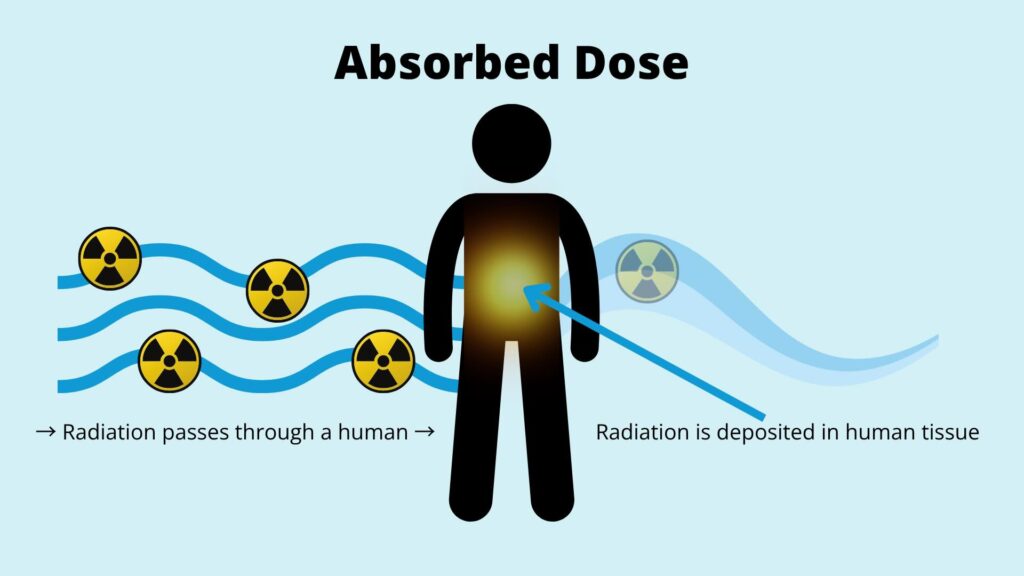
Detectors, however, don’t “feel” radiation like tissue does – they rely on luminescence or heating to measure the dose. That’s why tissue equivalence is so important: it ensures that the detector’s signal is proportional to what human tissue experiences.
In the plot below, four of the most widely used materials in commercial dosimetry are compared for photon energy response – and only one stays remarkably close to tissue equivalence across diagnostic and therapeutic ranges.
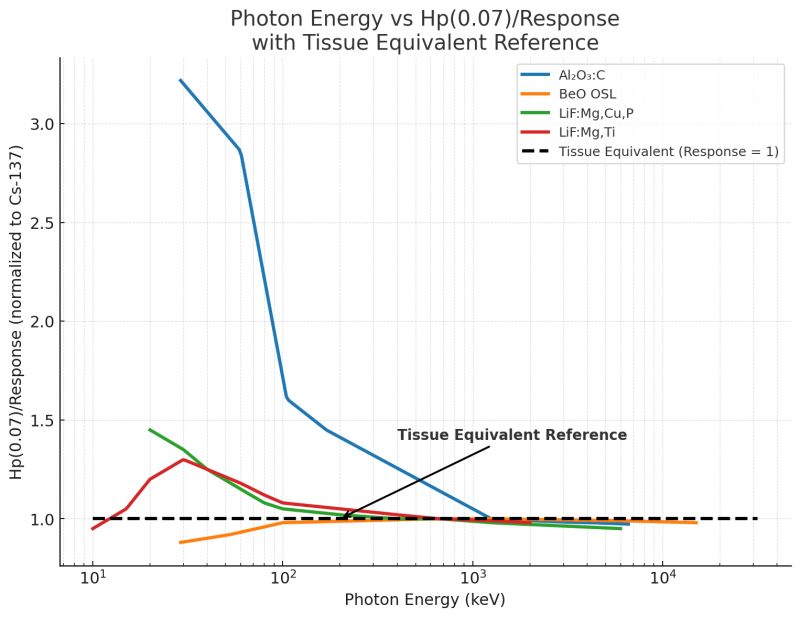
A detector material with a response ratio of 1.0 at a given photon energy is considered tissue-equivalent. Deviations from unity indicate either under-response (under-reporting dose) or over-response (over-reporting dose). The shape of this response curve across photon energies tells us how much correction or compensation is needed to achieve accurate results.
| Material | Type | Key Strengths | Limitations | Overall Summary |
|---|---|---|---|---|
| Al₂O₃:C (Aluminum Oxide: Carbon) | OSL | Extremely sensitive; can be re-read multiple times | Strong over-response at low photon energies (up to 3× tissue); requires complex corrections and filtering | High-performing but complex; best used with controlled energy sources |
| BeO (Beryllium Oxide) | OSL | Most tissue-equivalent OSL material; high sensitivity; minimal correction needed; stable and reliable over time | Costlier than LiF-based materials | Top performer for accuracy and simplicity; RDC’s chosen OSL material for modern systems |
| LiF:Mg,Cu,P (MCP) | TLD | Very sensitive; near tissue-equivalent; low fading and high reproducibility | Slight over-response at low energies | Excellent balance of accuracy and practicality; ideal for regulatory and medical monitoring |
| LiF:Mg,Ti (TLD-100) | TLD | Long-standing industry standard; flat response near unity; proven stability | Lower sensitivity than newer materials | Reliable benchmark material; ideal for calibration and long-term consistency |
Al₂O₃:C is one of the most common optically stimulated luminescence (OSL) materials used in commercial dosimetry. It offers excellent sensitivity and re-readability, but comes with a significant drawback: a strong over-response at low photon energies, sometimes up to three times that of tissue (Akselrod et al., 1999; Scarboro & Kry, 2013; Yukihara et al., 2008).
This over-response requires complex filtering, correction algorithms, and angular adjustments to approximate tissue equivalence. While workable, these corrections introduce uncertainty and complexity, particularly in mixed or variable energy environments like medical imaging suites.
BeO is the standout performer among OSL materials. It provides high sensitivity while maintaining a near-unity response across a broad energy range (Jahn et al., 2019).
Unlike Al₂O₃:C, BeO’s intrinsic properties make it more tissue-equivalent, reducing the need for correction factors or multi-element reconstruction. In real-world applications, this translates to simpler, more reliable dose measurement with fewer opportunities for calibration drift or energy bias.
At Radiation Detection Company, we’ve selected BeO OSL as a cornerstone material in our modern dosimetry systems – not just for its performance, but for its stability and practicality in long-term monitoring.
LiF:Mg,Cu,P is a highly sensitive thermoluminescent dosimeter (TLD) material with a slight over-response at low energies that stabilizes near unity as photon energy increases (Pradhan & Bhatt, 1989; Konnai et al., 2000s).
Because of its excellent reproducibility and low fading, LiF:Mg,Cu,P TLDs are ideal for monitoring a wide range of occupational and medical exposures. Their near-tissue response ensures dose accuracy without extensive algorithmic correction, which is why they remain a trusted choice for regulatory compliance and research dosimetry.
At Radiation Detection Company, we offer LiF:Mg,Cu,P TLD as a reliable dosimetry solution.
Often called the “workhorse” of radiation protection, TLD-100 (LiF:Mg,Ti) has been in use for decades. It exhibits a relatively flat response near unity across most diagnostic and protection energies, making it a dependable standard for historical comparison (Konnai et al., 2000s).
While newer materials may outperform it in sensitivity or reusability, its stable energy response continues to make TLD-100 an essential benchmark for calibrating and validating dose measurement systems.
Learn more about the differences between badge types with the Ultimate Radiation Badge Guide: Everything You Need to Know About Dosimetry Badges.
When a detector strays from tissue equivalence, it creates challenges:
A detector that naturally aligns with tissue equivalence, like BeO OSL, avoids many of these pitfalls. It allows the dosimetry system to produce accurate, direct readings with minimal compensation. In practice, that means faster reporting, greater confidence, and fewer administrative headaches for safety officers and RSOs.
In hospitals, research labs, and manufacturing facilities, dose measurement accuracy directly impacts regulatory compliance and worker safety.
When detector response deviates from tissue equivalence, even small energy biases can alter effective dose calculations. For instance:
Choosing a more tissue-equivalent material simplifies program management. It reduces the number of correction variables, makes calibration more stable, and improves trust in the reported dose of record.
That’s why, at Radiation Detection Company (RDC), we’ve chosen BeO OSL and LiF:Mg,Cu,P TLD. They offer high sensitivity, exceptional fade characteristics, and market-leading lower limit of detection performance while staying close to tissue equivalence, keeping dosimetry both accurate and practical.
Evaluate your organization’s compliance readiness with our Radiation Compliance Cheat Sheet.
When it comes to dose measurement, not all detector materials behave the same way. Each one has its own unique interaction with radiation, and that directly affects how accurately it reflects the dose received by tissue.
In personnel dosimetry, tissue equivalence serves as the gold standard. A detector that responds to radiation the same way human tissue does (a response ratio of 1.0) produces a more direct, reliable measure of dose. Detectors that over- or under-respond introduce complexity: they require energy corrections, filters, or software adjustments to reconcile the data with true biological exposure.
To illustrate these differences, the table below compares four of the most common materials used in commercial dosimetry today, highlighting their sensitivity, energy dependence, correction requirements, and tissue equivalence ratios.
| Material | Sensitivity | Energy Dependence | Correction Required | Tissue Equivalence Ratio (Response at 1.0 = Tissue) |
|---|---|---|---|---|
| BeO (OSL) | High | Minimal across diagnostic and therapeutic ranges | Low – typically negligible | ≈ 1.0 (Highly tissue-equivalent) |
| LiF:Mg,Ti (TLD-100) | Moderate | Relatively flat across most photon energies | Low – long-established calibration factors | ≈ 1.0–1.1 |
| LiF:Mg,Cu,P (TLD) | High | Slight over-response at low energies; stable near unity above 100 keV | Low to Moderate – generally stable with minimal correction | ≈ 1.1–1.2 |
| Al₂O₃:C (OSL) | Very High | Strong over-response at low photon energies (up to 3× tissue) | High – requires multi-element filters and correction algorithms | ≈ 2.0–3.0 at low energies |
BeO OSL remains the closest to true tissue equivalence, minimizing the need for correction or energy-dependent compensation. Al₂O₃:C, while highly sensitive, introduces significant over-response at diagnostic photon energies, requiring complex filtering. Both LiF-based TLDs perform reliably, with LiF:Mg,Cu,P offering enhanced sensitivity and stability suitable for high-precision applications.
Behind every dosimeter is a material with its own personality – some calm, some complicated.
The closer a detector’s response stays to 1.0, the simpler and more reliable the dose measurement becomes. Tissue equivalence isn’t just a theoretical benchmark – it’s what makes dosimetry trustworthy in the real world.
By choosing detectors that behave like tissue, we make dose reporting not only more accurate but more actionable. And that’s the foundation of every strong radiation safety program: one that protects people, ensures compliance, and upholds the integrity of the data we depend on.
Dose measurement quantifies the amount of radiation energy absorbed by tissue. Personnel dosimeters convert detector signals into dose equivalents that estimate biological effect, allowing compliance with regulatory limits and safety optimization.
Tissue equivalence ensures the detector responds to radiation the same way human tissue does. When a detector deviates from unity response, it either over- or under-reports exposure, requiring additional correction and increasing uncertainty.
OSL (optically stimulated luminescence) dosimeters use light to release trapped energy, allowing multiple readings, while TLDs (thermoluminescent dosimeters) require heating to release the stored signal.
For more details about the differences between each dosimeter type, check out this blog: TLD vs. OSL – Types of Dosimeters.
BeO (OSL) and LiF:Mg,Cu,P (TLD) are among the most commercially available tissue-equivalent materials. Both exhibit minimal energy dependence and high reproducibility, making them reliable for clinical, research, and industrial monitoring.
Select tissue-equivalent detectors, maintain consistent calibration protocols, and partner with dosimetry providers who validate performance across photon energies. Simplifying detector correction and calibration workflows enhances accuracy and confidence in dose records.
Energy dependence, detector material, angular response, calibration quality, and environmental conditions all play roles. The most controllable variable is detector selection – starting with a material that closely matches tissue response.

Learn how Radiation Detection Company’s easy-to-use dosimetry solutions can boost the efficiency of your practice.