
Dosimeter badge services for medical, dental, and veterinary businesses
Learn how Radiation Detection Company’s easy-to-use dosimetry solutions can boost the efficiency of your practice.

The four common types of radiation include alpha particles, beta particles, gamma rays, and neutron radiation. These forms of radiation differ in their mass, energy, and how deeply they are able to penetrate objects and humans. In this article, we will more deeply explore radiation basics, as well as alpha, beta, and gamma radiation.
As always, we hope you find this article informative, and we look forward to hearing your feedback!
In its simplest form, radiation is energy. Radiation comes from two sources:
Radiation travels from its source in two forms: either energized particles or energy waves. There are two kinds of radiation: non-ionizing radiation and ionizing radiation.
Non-ionizing radiation has enough energy to move atoms in a molecule around (or at the very least cause them to vibrate). However, it does not have enough energy to actually remove electrons from atoms. Examples of non-ionizing radiation are radio waves, visible light, and microwaves.
Humans are exposed to low levels of non-ionizing radiation daily. Exposure to intense and direct amounts of non-ionizing radiation can cause damage to tissue due to heat. This is not at all common and is really only a concern for personnel who work on large sources of non-ionizing radiation devices and instruments.
Ionizing radiation has so much energy that it can knock electrons out of atoms. This process is known as ionization. Ionizing radiation poses a serious risk to the health of living things due to its potential to damage tissue and DNA in genes. Ionizing radiation can come from x-ray machines, cosmic particles (particles from outer space), and radioactive elements.
Radioactive elements emit ionizing radiation as their atoms undergo radioactive decay, a process in which a radioactive atom spontaneously gives off radiation in the form of energy or particles in order to reach a more stable state. All types of nuclear radiation are ionizing radiation, while the reverse is not always true.
Alpha particles - denoted by symbol α - are positively charged, and are made up of two protons and two neutrons from the atom’s nucleus (identical to a helium-4 nucleus). Alpha particles come from the decay of the heaviest radioactive elements, such as uranium, radium, thorium, and polonium - a process known as alpha decay. Although alpha particles are quite energetic, they are so heavy that they use up all of their energy over very short distances. This makes then incapable of traveling very far from the atom.
How a person is exposed to alpha particles determines how dangerous the radiation can be. Alpha particles have low penetrating power, meaning they lack the energy to penetrate the outer layer of skin. This makes exposure to the exterior of the body a non-issue. However, inside the body they can be very harmful. If an alpha-emitter is inhaled, swallowed, or gets into the body through a cut, it can damage living tissue (which is extremely sensitive). This makes these large, heavy particles more dangerous than other types of radiation, with alpha radiation resulting in more severe damage to cells and DNA.

Beta particles - denoted by symbol β - are small, fast-moving particles with a negative electrical charge. They are emitted from an atom’s nucleus during beta decay. Beta decay generally occurs in nuclei that have too many neutrons to achieve stability. These particles are emitted by specific unstable atoms like hydrogen-3 (tritium), carbon-14, and strontium-90.
Beta particles have a higher penetrating power than alpha particles, but they are less damaging to living tissue and DNA. This is because the ionizations they produce are more spaced out. Beta particles can travel further in air than alpha particles can, but they can be easily stopped by a layer of clothing or by a thin layer of substances (like aluminum). However, some beta particles are capable of penetrating the skin and causing damage (i.e. skin burns). Like alpha-emitters, beta-emitters are their most dangerous when they are inhaled or swallowed.
Gamma rays - denoted by symbol γ - are weightless packets of energy called photons. Unlike alpha and beta particles - which have both energy and mass - gamma rays are purely energy. Gamma rays are similar to visible light but have much higher energy. Gamma rays are emitted during radioactive decay (like alpha or beta particles). They are also produced by the hottest and most energetic objects in the universe (i.e. neutron stars and pulsars, supernova explosions, and regions around black holes). They have the shortest wavelength (even shorter than x-rays) and most energy of any wave in the electromagnetic radiation spectrum. Although gamma rays and x-rays have the same basic properties, they come from different parts of the atom. Gamma rays originate inside the nucleus, while x-rays are emitted from processes outside the nucleus.
Photon beam radiation therapy is a type of radiation therapy that uses x-rays or gamma rays. These rays come from a special machine called a linear accelerator (linac). The radiation dose is delivered at the surface of the body and penetrates the tumor and cells in the body. While photon beam radiation therapy can be confused with proton beam therapy, the two are different.
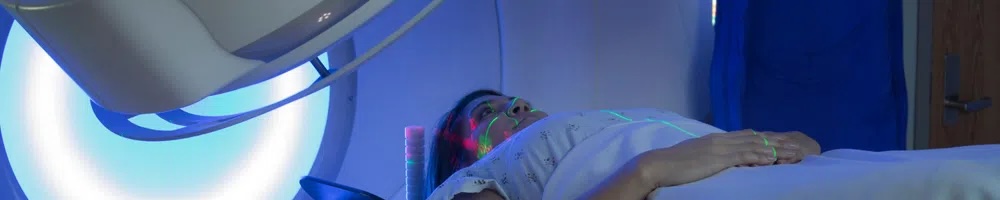
Gamma rays pose a severe radiation hazard for the entire human body. They can easily penetrate the barriers that are able to stop alpha and beta particles (like clothing and skin). The penetrating power of gamma rays is so strong, that a barrier of lead several inches thick, or a few feet of concrete are required to stop them. Gamma rays are able to completely pass through the human body, causing ionizations that damage tissue and DNA as they travel.
Neutron radiation is distinct from alpha, beta, and gamma radiation. This is a form of ionizing radiation that presents as free neutrons (which are unstable). Nuclear fission or nuclear fusion generally causes the release of free neutrons, which then react with nuclei of other atoms. This reaction forms new nuclides, which can trigger even further neutron radiation. Neutrons are very penetrating particles. Several feet of concrete or other material rich in hydrogen (like water) are required to stop this penetration. Neutrons are a radiation hazard for the entire body, as they have the ability to interact with tissues in the body and potentially cause damage.
Radiation Detection Company (RDC) is dedicated to your safety and the safety of all of your employees. We have 75 years of experience providing quality dosimetry solutions to over 31,000 companies worldwide.
To see which of RDC’s radiation safety products are right for your department or organization, please visit our Solutions page to view our full suite of offerings. We offer a wide range of affordable and comprehensive solutions to fit the needs of both large and small organizations.
Have a question that we did not address in this article? Please reach out to our Customer Care team, and one of our specialists will be happy to support you.

Learn how Radiation Detection Company’s easy-to-use dosimetry solutions can boost the efficiency of your practice.